What is a primate?
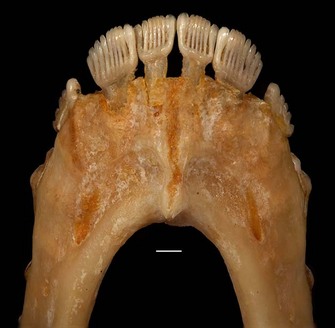
The group of fossils that is the focus of debates over primate origins is called the plesiadapiforms. This is an incredibly diverse group including more than 140 named species arranged into 11 different families. The first record of plesiadapiforms appears just as the non-avian dinosaurs were going extinct about 65 million years ago, near the beginning of the Paleocene. Some plesiadapiforms persist well into the Eocene, with the last species going extinct around 37 million years ago (Silcox & Gunnell, 2008). Over the course of its more than 25-million-year history, the group underwent an impressive adaptive radiation, producing forms with very distinctive dental adaptations including weird, multi-cusped upper incisors, and a diversity of very specialized lower premolars (Figure 2).

The most comprehensive analysis to date of the relationships among plesiadapiforms, primates, and closely related mammals is by Bloch and colleagues (2007; Figure 3). The results of this analysis support the idea that plesiadapiforms are more closely related to primates than to any other group. These authors argued that plesiadapiforms should therefore be considered stem primates; they adopted the name Euprimates (Hofstetter, 1977) for living primates and any fossil forms that exhibit all of the features of modern primates listed above. It is worth noting, however, that not all researchers are convinced by Bloch et al.'s (2007) results. These workers generally equate the Order Primates with Euprimates, excluding plesiadapiforms from the order. This leads to a very different conception of what constitutes primate origins than discussed here (e.g., Soligo & Martin, 2007), with the order not appearing until nearly 10 million years later, when the first forms that share traits such as the postorbital bar and nails on most digits (e.g., adapoids and omomyoids) are first found in the fossil record (e.g., Rose et al., 2012).
The broader relationships of Primates in Mammalia
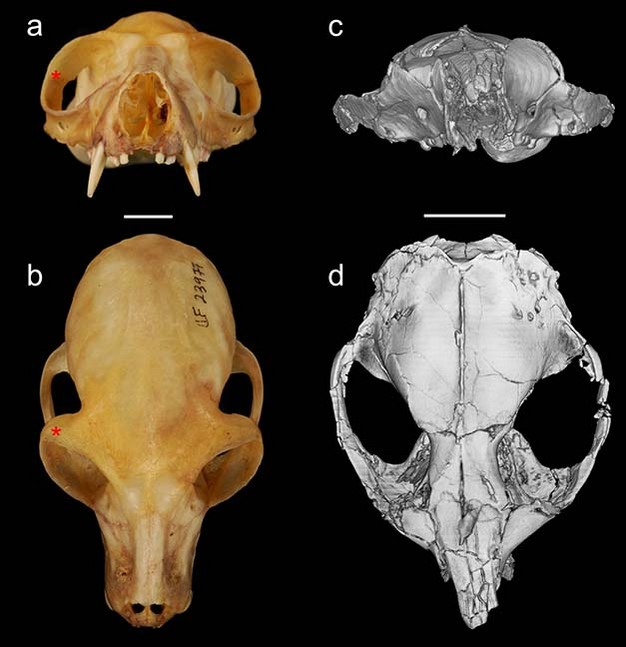
Although there are continuing disagreements about where to draw the primate/non-primate line in the fossil record, more consensus exists about the identity of the closest living relatives of primates. Molecular analyses of mammalian relationships have fairly consistently placed primates in a group called Euarchonta with two other living orders: Scandentia and Dermoptera (Springer et al., 2004). Scandentians are small, quadrupedal animals that live in Southeast Asia. They are more commonly referred to as treeshrews, although not all of them live in the trees, and taxonomically they are not shrews. From 1922 (Carlsson, 1922) until 1980, treeshrews were often classified as primates. This idea was effectively refuted in a landmark edited volume (Luckett, 1980) that assessed the primate hypothesis using multiple lines of evidence. Even though trees shrews are no longer considered to be primates, familiarity with them is very important for researchers interested in primate origins because they provide the best living models for the earliest members of the Order. Indeed, the most primitive plesiadapiform known from a reasonably complete skeleton (Dryomomys szalayi) closely resembles the pen-tailed treeshrew, Ptilocercus lowii (Figure 4), below the head (Bloch et al., 2007). These similarities suggest that the first primate probably looked quite a bit like Ptilocercus, and supports the idea (Szalay & Drawhorn, 1980) that primates evolved from an already arboreal ancestor.

Where, when, and why did primates evolve?
The earliest known plesiadapiforms are placed in the genus Purgatorius. Although there is a fragment of a tooth for this genus that has been described as coming from the same deposit as a Triceratops skeleton (Van Valen & Sloan, 1965), this deposit includes a mixture of material of different ages (Clemens, 2004). More firmly dated specimens are known from the earliest part of the Paleocene, however, so it is clear that even if Purgatorius did not overlap in time with the non-avian dinosaurs, it was one of the first groups to exploit the new opportunities created by their extinction (Johnston & Fox, 1984; Clemens, 2004).
Although the age and primitive morphology of Purgatorius would suggest an origin of primates around 65 million years ago, molecular dates for the origin of the order are generally at least 10 million years earlier, well before the Cretaceous-Tertiary boundary (Springer et al., 2003). If this is the case, we may be missing or not recognizing a significant part of the early history of the order. Alternatively, this discrepancy could reflect problems with the assumptions underlying molecular clock models, such as constancy of evolutionary rate (Ho, 2008), and the difficulty of calibrating the clock (e.g., see discussion in Stauffer et al., 2001).
While the Southeast Asian location of the closest living relatives of primates might suggest an Asian origin for the order (Beard, 2004), the North American location of most primitive plesiadapiforms supports a North American origin instead (Bloch et al., 2007). This may be a product, however, of much greater sampling of the fossil record in North America. Indeed, there is now a relatively primitive plesiadapiform known from Asia (Asioplesiadapis youngi Fu et al., 2002; see discussion in Silcox, 2008), which suggests that further discoveries on that continent may make it seem a more plausible place of origin for Primates.
In terms of why primates evolved, most of the scenarios for primate origins that discuss plesiadapiforms (rather than those restricted to Euprimates) suggest a relationship to diet. Szalay, in his landmark 1968 paper, "The beginnings of Primates," wrote: "It is only an increasing occupation of feeding on fruits, leaves, and other herbaceous matter that explains the first radiation of primates" (p. 32), and Sussman and Raven (1978) included plesiadapiforms in their proposed co-evolutionary scenario between primates and flowering plants. Eriksson et al. (2000) document an increase in the volume of fruit and proportion of animal-dispersed flowering plants through the late Cretaceous into the Paleocene; early primates may have been one of the forces driving this change. These suggestions may seem surprising in light of the small, rather pointy teeth of Purgatorius (Figure 2a). Certainly in comparisons with modern primates this morphology would suggest a diet richer in insects than in plant materials (Kay & Cartmill, 1977). However, it is important to note that the features we use to differentiate Purgatorius and the other early plesiadapiforms from contemporaneous small mammals (e.g., broad talonid basins) are precisely those which can be related to a more diverse diet. So while we still have much to learn about the ecological profile of the earliest primates, a shift to a more omnivorous diet may have been one part of their success. A diffuse co-evolutionary relationship with flowering plants may have subsequently played a critical role in shaping the diversification of plesiadapiforms throughout the Paleocene (Sussman, 1991; Bloch et al., 2007).References and Recommended Reading
Beard, K. C. Gliding Behavior and palaeoecology of the alleged primate family Paromomyidae (Mammalia, Dermoptera). Nature 345, 340-341 (1990).
Beard, K. C. The Hunt for the Dawn Monkey: Unearthing the Origins of Monkeys, Apes, and Humans. Los Angeles: University of California Press, (2004).
Bloch, J. I. & Boyer, D. M. Grasping Primate Origins. Science 298, 1606-1610 (2002).
Bloch, J. I., Silcox M. T., et al. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proceedings of the National Academy of Science 104, 1159-1164 (2007).
Carlsson, A. Uber die Tupaiidae und ihre Beziehungen zu den Insectivora und den Prosimiae. Acta Zoologica 3, 227-270 (1922).
Cartmill, M. Rethinking Primate Origins. Science 184, 436-443 (1974).
Clemens, W. A. Purgatorius (Plesiadapiformes, Primates?, Mammalia), a Paleocene immigrant into Northeastern Montana: stratigraphic occurrences and incisor proportions. In Fanfare for an Uncommon Paleontologist: Papers in Honor of Malcolm C. McKenna. eds. Dawson, M. R. & Lillegraven J. A. (Pittsburgh: Bulletin of the Carnegie Museum of Natural History, 36, 2004) 3-13.
Eriksson, O., Friis, E. M. et al. Seed size, fruit size, and dispersal systems in angiosperms from the Early Cretaceous to the Late Tertiary. American Naturalist 156, 47-58 (2000).
Fu, J.-F., Wang, J.-W. et al. The new discovery of the Plesiadapiformes from the early Eocene of Wutu Basin, Shandong Province. Vertebrata PalAsiatica 40, 219-227 (2002).
Ho, S. The molecular clock and estimating species divergence. Nature Education 1 (2008) http://www.nature.com/scitable/topicpage/the-molecular-clock-and-estimating-species-divergence-41971
Hoffstetter, R. Phylogénie des Primates. Bulletins et Mémoires de la Société d'Anthropologie de Paris t4, XIII, 327-346 (1977).
Janečka, J. E., Miller, W., et al. Molecular and genomic data identify the closest living relative of Primates. Science 318, 792-794 (2007).
Kay R. F. & Cartmill M. Cranial morphology and adaptations of Palaechthon nacimienti and other Paromomyidae (Plesiadapoidea, ?Primates), with a description of a new genus and species. Journal of Human Evolution 6, 19-53 (1977).
Kay, R. F., Thorington, R. W. et al. Eocene plesiadapiform shows affinities with flying lemurs not Primates. Nature 345, 342-344 (1990).
Luckett, W. P. Comparative Biology and Evolutionary Relationships of Tree Shrews. New York: Plenum Press (1980).
MacPhee, R. D. E., Cartmill, M. et al. Craniodental morphology and relationships of the supposed Eocene dermopteran Plagiomene (Mammalia). Journal of Vertebrate Paleontology 9, 329-49 (1989).
Martin, R. D. Towards a new definition of Primates. Man 3, 377-401 (1968).
Novacek, M. J. Mammalian phylogeny: shaking the tree. Nature 356, 121-125 (1992).
Rose, K.D. , Chew, A.E., et al. Earliest Eocene mammalian fauna from the Paleocene-Eocene thermal maximum at Sand Creek Divide, Southern Bighorn Basin, Wyoming. University of Michigan Papers on Paleontology 36, 1-122. (2012)
Sargis, E.J. New views on tree shrews: the role of tupaiids in primate supraordinal relationships. Evolutionary Anthropology 13, 56-66 (2004).
Silcox, M. T. New discoveries on the middle ear anatomy of Ignacius graybullianus (Paromomyidae, Primates) from ultra high resolution X-ray computed tomography. Journal of Human Evolution 44, 73-86 (2003).
Silcox, M. T. The biogeographic origins of Primates and Euprimates: East, West, North, or South of Eden? In Mammalian Evolutionary Morphology: a Tribute to Frederick S. Szalay. eds. Dagosto M. & Sargis, E. J. (New York: Springer-Verlag, 2008) 199-231.
Silcox, M. T. & Gunnell, G. F. Plesiadapiformes. In Evolution of Tertiary Mammals of North America Vol. 2: Marine Mammals and Smaller Terrestrial Mammals. Eds. Janis C. M., Gunnell, G. F. & Uhen, M. D. (Cambridge: Cambridge University Press, 2008) 207-238.
Silcox M. T., Bloch J. I., et al. Euarchonta. In The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades. Eds. Rose K.D. & Archibald J. D. (Baltimore: Johns Hopkins University Press 2005) 127-144.
Silcox M. T., Bloch J. I., et al. Cranial anatomy of Paleocene and Eocene Labidolemur kayi (Mammalia: Apatotheria) and the relationships of the Apatemyidae to other mammals. Zoological Journal of the Linnean Society 160, 773-825 (2010).
Soligo, C. & Martin, R.D. The first primates: a reply to Silcox et al. Journal of Human Evolution 53, 325-328 (2007).
Springer M. S., Murphy W. J., et al. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proceedings of the National Academy of Science 100, 1056-1061 (2003).
Springer M. S., Stanhope M. J. et al. Molecules consolidate the placental mammal tree. Trends in Ecology and Evolution 19, 430-438 (2004).
Stauffer, R.L., Walker, A. et al. Human and ape molecular clocks and constraints on paleontological hypotheses. The Journal of Heredity 92,469-474 (2001).
Sussman R.W. Primate origins and the evolution of angiosperms. American Journal of Primatology 23, 209-223 (1991).
Sussman R. W. & Raven P. H. Pollination of flowering plants by lemurs and marsupials: a surviving archaic coevolutionary system. Science 200, 731-736 (1978).
Szalay, F. S. The beginnings of primates. Evolution 22, 19-36 (1968).
Szalay F. S. & Drawhorn G. Evolution and diversification of the Archonta in an arboreal milieu. In Comparative Biology and Evolutionary Relationships of Tree Shrews. ed. Luckett , W. P. (New York: Plenum Press ,1980) 133-169.
Van Valen L. M. & Sloan R. E. The earliest Primates. Science 150, 743-745 (1965).



Nenhum comentário:
Postar um comentário
Observação: somente um membro deste blog pode postar um comentário.