Estimating the evolutionary rates in mosasauroids and plesiosaurs: discussion of niche occupation in Late Cretaceous seas
Author and article information
Abstract
Observations of temporal overlap of niche occupation
among Late Cretaceous marine amniotes suggest that the rise and
diversification of mosasauroid squamates might have been influenced by
competition with or disappearance of some plesiosaur taxa. We discuss
that hypothesis through comparisons of the rates of morphological
evolution of mosasauroids throughout their evolutionary history with
those inferred for contemporary plesiosaur clades. We used expanded
versions of two species-level phylogenetic datasets of both these
groups, updated them with stratigraphic information, and analyzed using
the Bayesian inference to estimate the rates of divergence for each
clade. The oscillations in evolutionary rates of the mosasauroid and
plesiosaur lineages that overlapped in time and space were then used as a
baseline for discussion and comparisons of traits that can affect the
shape of the niche structures of aquatic amniotes, such as tooth
morphologies, body size, swimming abilities, metabolism, and
reproduction. Only two groups of plesiosaurs are considered to be
possible niche competitors of mosasauroids: the brachauchenine
pliosaurids and the polycotylid leptocleidians. However, direct evidence
for interactions between mosasauroids and plesiosaurs is scarce and
limited only to large mosasauroids as the predators/scavengers and
polycotylids as their prey. The first mosasauroids differed from
contemporary plesiosaurs in certain aspects of all discussed traits and
no evidence suggests that early representatives of Mosasauroidea
diversified after competitions with plesiosaurs. Nevertheless, some
mosasauroids, such as tylosaurines, might have seized the opportunity
and occupied the niche previously inhabited by brachauchenines, around
or immediately after they became extinct, and by polycotylids that
decreased their phylogenetic diversity and disparity around the time the
large-sized tylosaurines started to flourish.
Cite this as
2020. Estimating the evolutionary rates in mosasauroids and plesiosaurs: discussion of niche occupation in Late Cretaceous seas. PeerJ 8:e8941 https://doi.org/10.7717/peerj.8941
Main article text
Introduction
Marine
amniotes underwent changes in the relative contribution of particular
clades to overall functional disparity near the Early-Late Cretaceous
transition (Stubbs & Benton, 2016). The contribution of plesiosaurs visibly decreased while that of mosasauroids was rising (Stubbs & Benton, 2016:
Fig. 3), suggesting that in addition to the extinction of ichthyosaurs
and significant reorganization of marine environments, competition
between plesiosaurs and mosasauroids might have played a role. Brief
discussion of such possibility has previously been provided by Schumacher (2011) following discovery of a russellosaurinan mosasauroid co-occurring with the last brachauchenine pliosaurids.
Origins,
diversification, and decline of particular clades of aquatic Mesozoic
amniotes are commonly linked to climatic changes and environmental
volatility (e.g., Benson & Druckenmiller, 2014; Fischer et al., 2016; Tennant, Mannion & Upchurch, 2016).
The same applies for mosasauroid squamates whose early evolution in the
early Late Cretaceous was hypothesized to have been primarily driven by
high marine productivity associated with warm climate and high sea
stands (Polcyn et al., 2014).
The factors influencing large-scale biotic events, such as turnovers,
however, are often tricky to grasp in full, especially if they involve
clade-specific interactions, such as competition, that depend on many
biological factors (see general studies by Benton, 1983; Benton, 1987; Benton, 1991; and, for example McGowan & Dyke, 2007; Butler et al., 2009; Benson et al., 2014 for detailed assessments of pterosaur-bird competitiveness hypothesis).
This
study is aimed to discuss the evolutionary history of mosasauroids, a
clade of aquatic squamates known exclusively from the Upper Cretaceous
strata (e.g., Polcyn et al., 2014; Simões et al., 2017; Madzia & Cau, 2017),
through the inference of their rates of morphological evolution.
Specifically, the evolutionary rates and traits of mosasauroids are
compared to those of plesiosaurs, a successful clade of aquatic amniotes
that originated in the Late Triassic and disappeared at the end of the
Cretaceous (e.g., Ketchum & Benson, 2010; Benson & Druckenmiller, 2014; Wintrich et al., 2017).
Increased
dynamics of phenotypic evolution in adaptive radiations is commonly
linked with interactions of sympatric species that lead to the
phenomenon Darwin (1859) called ‘divergence of character’ (now termed ‘character displacement’; Brown & Wilson, 1956; see also Pfennig & Pfennig, 2010),
which maintains that differences in traits of species with similar
phenotypes appear to minimize competitive interactions between their
populations. This is a ‘small-scale’ process that is well-documented on
closely related species (e.g., Sætre et al., 1997; Grant & Grant, 2010).
On a larger, macroevolutionary scale, however, the contribution of
competitive interactions still remains somewhat unclear (e.g., Tobias et al., 2014)
and contrasting with respect to particular types of traits, such as
those associated with resource-use and those involved in social
interactions (e.g., Drury et al., 2018).
In wholly extinct distantly-related clades with some similar traits and
comparable ecologies that at least partially overlapped in time and
space, such as mosasauroids and plesiosaurs, the role of competition is
particularly difficult to infer. Any assessments must be based on data
obtained from a highly incomplete fossil record. We assume that if
larger-scale competitive interactions between mosasauroids and
plesiosaurs took place at a certain time, signals of these interactions
might be noticeable in the evolutionary rates of the competing lineages.
No connection between evolutionary rates and competitive interactions
has ever been tested and higher/lower rates of divergence in these
clades may naturally have other causes as well (e.g., environmental).
Nevertheless, estimations of the evolutionary rates of the mosasauroids
and plesiosaurs that co-occurred and shared a number of traits
suggesting that they occupied similar or the same niches, and discussion
of traits that are tightly related to niche occupation, could initiate
further clade- or trait-specific studies that will elucidate the
patterns of niche occupation in Late Cretaceous seas.
In
order to assess the rates of morphological evolution among mosasauroids
and among different plesiosaur clades during the mid- to Late
Cretaceous, we used two species-level phylogenetic datasets, each
modified to reflect the current knowledge regarding the morphological
traits within both these groups, updated them with stratigraphic
information, and analyzed using the Bayesian inference to reconstruct
the tree topologies for both clades and times and rates of divergence
for their particular branches.
Following
the inferred results and comparisons of traits related to niche
occupation, possible competitive interactions of mosasauroids and
plesiosaurs are discussed within the criteria of these three hypotheses:
-
First mosasauroids diversified following competition with plesiosaurs.
-
At least some mosasauroids competed with contemporary plesiosaurs or seized the opportunity and occupied their niches when they were in demise or became extinct.
-
The fates of plesiosaurs and mosasauroids were independent of each other (no suggested competitive interactions between mosasauroids and plesiosaurs).
Methods
Bayesian inference
Both mosasauroid and plesiosaur phylogenetic datasets were analyzed using the protocol discussed in Madzia & Cau (2017),
integrating the morphological data matrices with absolute ages of the
least inclusive stratigraphic range including each terminal unit. The
Sampled Ancestor Fossilized Birth Death Skyline Model (SAFBD) of Gavryushkina et al. (2014) and Gavryushkina et al. (2017) implemented in BEAST 2.4.4. (Drummond et al., 2012; Bouckaert et al., 2014) was used as tree model. Since the character matrices did not include autapomorphies of the sampled taxa, the Lewis’s (2001)
model was conditioned to variable characters only using the
implementation included in BEAST 2.4.4. Stratigraphic information for
the mosasauroid and plesiosaur taxa was taken from the literature (Polcyn et al., 2014; Fischer et al., 2017;
respectively), and converted to geochronological ages. Stratigraphic
data and age constraints for each terminal were obtained from the
Paleobiology Database (http://paleobiodb.org/), checked against the International Chronostratigraphic Chart (v2019/05; http://stratigraphy.org/), and included as uniform prior for tip-dating (Supplemental Information I).
The
impact of using (or omitting) age priors incorporating stratigraphic
uncertainty in tip-dating has only recently been addressed (Barido-Sottani et al., 2019; Cau, 2019). Note that in their Bayesian analysis of Mosasauroidea, Madzia & Cau (2017)
used a punctiform age prior for each terminal taxon (i.e., the mean
value of the shortest age range encompassing the stratigraphic
uncertainty), thus they did not incorporate age uncertainty in tree
reconstruction. Such a strategy may arbitrarily set the age of several
taxa sharing the same stratigraphic uncertainty to an identical value,
thus enforcing a strictly cladogenetic pattern for their relationships
even under the SAFBD model, and biasing tree reconstruction favoring
longer ghost lineages. Furthermore, punctiform tip-dating priors may
lead to inflated divergence rates for taxonomic units scored from
multiple non-contemporary specimens (see Cau, 2019).
The
following protocol was used for both mosasauroid and plesiosaur
datasets. Each BEAST analysis involved 3 replicate runs (with different
random starting trees and random number seeds). Each of the 3 replicate
runs involved 10 million steps with sampling every 1,000 generations,
with a burnin of 4 million steps. This protocol is similar to the one
followed by Simões et al. (2017)
but used an additional independent run for each analysis (i.e., three
instead of two) and set a more conservative burnin (40% instead of 25%).
We used Tracer 1.5 (Rambaut & Drummond, 2009)
to determine whether the runs reached stationary phase, and to assess
convergence of the independent runs. The post-burnin parameter and tree
samples were retained for the analysis and concatenated using
LogCombiner in the BEAST package. Estimates (mean and 95% highest
posterior density) for all numerical parameters were generated using
Tracer 1.5 (Rambaut & Drummond, 2009).
We used the MCCT to reconstruct the cladogenetic events (median age)
and to infer the divergence rate (the amount of morphological change per
branch relative to the whole topology) for both clades. Note that the
absolute rate values are inversely related to the sample size
(i.e., rate value in a branch is proportional to the probability of
sampling each state transition of the clade history in that branch);
thus, direct comparisons between the mosasauroid and plesiosaur rate
values is meaningless. Given the rate distribution inferred along the
MCCT in the two clades, we here define ‘high rates’ all those values
equal or higher than the value at the 75 percentile in each rate
distribution.
The mosasauroid matrix was acquired from Simões et al. (2017), which is a recent version of the dataset first introduced in Bell’s (1993) PhD thesis and published by Bell (1997). The recent version of Simões et al. (2017) was further modified with respect to some taxon names (as in Madzia & Cau, 2017). In sum, the dataset consists of 44 operational taxonomic units (OTUs) analyzed using 125 characters (see Supplemental Information II for BEAST file, Supplemental Information III for NEXUS file, and Supplemental Information IV for character list).
The phylogenetic assessment of Plesiosauria was performed using a modified version of the dataset first assembled by Benson & Druckenmiller (2014). We first took a recent version of that dataset, published by Madzia, Sachs & Lindgren (2019),
and updated it based on personal observations and recently published
literature, to include representatives of distinctive plesiosaur clades.
The changes include: modifications to the scores of Brancasaurus brancai and ‘Gronausaurus wegneri’ as in Sachs, Hornung & Kear (2016); addition of Lagenanectes richterae from Sachs, Hornung & Kear (2017); addition of Nakonanectes bradti, Albertonectes vanderveldei, Aristonectes quiriquinensis, Elasmosaurus platyurus, ‘Hydralmosaurus serpentinus’, Mauisaurus haasti, ‘Libonectes’ atlasense, Terminonatator ponteixensis, Tuarangisaurus keyesi, Zarafasaura oceanis, Kawanectes lafquenianum, and Vegasaurus molyi from Serratos, Druckenmiller & Benson (2017); addition of Neusticosaurus pusillus and Nothosaurus marchicus, and modifications to the scores of Yunguisaurus liae and Pistosaurus OTUs as in Wintrich et al. (2017); addition of Acostasaurus pavachoquensis, ‘Kronosaurus’ boyacensis, and Sachicasaurus vitae from Páramo-Fonseca, Benavides-Cabra & Gutiérrez (2018), with amended scores for A. pavachoquensis and S. vitae as in Páramo-Fonseca, Benavides-Cabra & Gutiérrez (2019); modifications to the character scores of Thililua longicollis and addition of Eopolycotylus rankini, Manemergus anguirostris, Dolichorhynchops tropicensis, Georgiasaurus penzensis, Dolichorhynchops sp. (specimen ROM 29010), Dolichorhynchops herschelensis, Sulcusuchus erraini, and Mauriciosaurus fernandezi following Fischer et al. (2018), with amended scores for Trinacromerum bentonianum, Dolichorhynchops osborni, Dolichorhynchops bonneri, Mauriciosaurus fernandezi, and Polycotylus latipinnis as in Morgan & O’Keefe (2019); addition of Styxosaurus snowii from Sachs, Lindgren & Kear (2018); and modifications to the scores of Kronosaurus queenslandicus and Stenorhynchosaurus munozi following Holland (2018) and Páramo-Fonseca, Benavides-Cabra & Gutiérrez (2019).
It
is essential to note that although the elasmosaurid phylogenetic
relationships were a subject of several recent papers (e.g., Otero, 2016; Sachs, Hornung & Kear, 2016; O’Gorman et al., 2017; Sachs & Kear, 2017; Serratos, Druckenmiller & Benson, 2017; Sachs, Lindgren & Kear, 2018; O’Gorman et al., 2019),
interpretations of morphologies observed in some elasmosaurid specimens
differ between these studies. See, for example, conflicting scores for ‘Libonectes’ atlasense in Sachs & Kear (2017) and Serratos, Druckenmiller & Benson (2017), and for Styxosaurus snowii in Serratos, Druckenmiller & Benson (2017) and Sachs, Lindgren & Kear (2018).
We have not studied these taxa in person; as such, we had to choose
between scores provided in other publications. We decided to adopt those
scores that derive from more recent studies in which the taxa were
assessed based on direct observations. For that reason, ‘Libonectes’ atlasense is here scored as in Serratos, Druckenmiller & Benson (2017) and Styxosaurus snowii as in Sachs, Lindgren & Kear (2018).
Naturally, such differences in interpretations of character states
might have an impact on inferred evolutionary rates. Nevertheless, our
decisions should not have any impact on the findings of the present
study as Elasmosauridae is of marginal importance here.
Finally, we have also modified several character states of Anguanax zignoi
based on personal observations of the type specimen (3: 0→?; 4: [12]→?;
121: 0→?; 137: 0→?; 150: 1→?; 207: [01]→?; 270: 1→0) and re-scored Megacephalosaurus eulerti for character 27 (1→0). This score has been already advocated by Madzia, Sachs & Lindgren (2019: p. 1208) but the character was erroneously scored as ‘1’ rather than ‘0’ in that study.
Additionally, we have modified several character definitions in the character list of Benson & Druckenmiller (2014):
Character 25.
The character description was changed from “Maxilla, posterior extent
of maxillary tooth row” to “Maxilla and dentary, posterior extent of
maxillary tooth row”; after Serratos, Druckenmiller & Benson (2017).
Character 138. As noted by Madzia, Sachs & Lindgren (2019),
the current state definitions for character 138 are problematic because
they do not cover all plesiosaurs. In the original character list of Benson & Druckenmiller (2014),
state ‘0’ was defined as codable for taxa with 12–17 maxillary teeth,
state ‘1’ for taxa with 20–25 maxillary teeth, and state ‘2’ for taxa
with more than 28 maxillary teeth. However, the brachauchenine
pliosaurid Megacephalosaurus eulerti was shown to possess 18
teeth in the right and 19 in the left maxilla, thus falling between
states ‘0’ and ‘1’. Two options were considered for M. eulerti:
to score it as ‘0’, extending the state to cover taxa with 12–19
maxillary teeth, and as ‘1’, extending the state to cover taxa with
18–25 teeth in their maxillae. Madzia, Sachs & Lindgren (2019)
used both these options and explored the effects of such settings.
Considering that the last brachauchenines have reduced numbers of teeth
in their jaws (Madzia, Sachs & Lindgren, 2019),
scoring these taxa in the same way as their older relatives (that fall
near the upper boundary of state ‘1’) might hinder the inference of some
potential phylogenetic signal. Therefore, in this study, state ‘0’
covers taxa with 12–19 maxillary teeth, state ‘1’ covers taxa with 20–27
maxillary teeth (note that the upper boundary was extended to eliminate
the gap between states ‘1’ and ‘2’), and state ‘2’ covers taxa with at
least 28 maxillary teeth.
Character 139.
State ‘2’ (“intermediate between states 0 and 1, with a flattened
labial surface, but this surface [is] not substantially expanded
anteroposteriorly [= subtrihedral]”) was added after Benson et al. (2013). Even though Benson et al. (2013)
described the state ‘2’ as “intermediate”, the morphological transition
from “round or sub-rounded” (‘0’) to “sub-triangular [= trihedral]”
(‘1’) does not need to have the appearance of ‘2’. Later, Serratos, Druckenmiller & Benson (2017) used the dataset of Benson & Druckenmiller (2014)
to infer the interrelationships of elasmosaurids and modified character
139 to include another new state (‘2’): “suboval”. However, in their
data matrix, this state was scored as ‘3’. In this study, the state ‘2’
is equivalent to state ‘2’ of Benson et al. (2013), and state ‘3’ follows the new “suboval” state introduced by Serratos, Druckenmiller & Benson (2017).
Nevertheless, the perception of what is “sub-rounded” (‘0’) and what
“suboval” (3) may be partially dependent on subjective criteria. As
such, future larger-scale phylogenetic studies of Plesiosauria should
probably quantify the difference (for example, using the
‘width-to-length ratio’ [WLR] of Madzia (2016)
or similarly defined parameter). Due to the lack of apparent
transitional nature of particular character states, this character
should stay unordered in parsimony analyses.
Character 153. State ‘3’ (“even longer, corresponding to the ‘can’ shaped morphology of Otero et al. (2016a) or ‘elongate’ morphology of O’Keefe & Hiller (2006)”) was added after Serratos, Druckenmiller & Benson (2017). We did not modify the state definition though, again, its quantification would enable to maintain an unambiguous application.
Character 248.
The character description was changed from “Propodials, angle between
long axes of epipodial facets in dorsal view” to “Humerus, angle between
long axes of epipodial facets in dorsal view”; after Serratos, Druckenmiller & Benson (2017).
The full dataset consists of 125 OTUs analyzed using 270 characters (see Supplemental Information V for BEAST file, Supplemental information VI for NEXUS file, and Supplemental information VII for character list). This is the largest dataset used in phylogenetic assessment of Plesiosauria published to date.
Reconstruction of geographic origin
A
detailed assessment of the historical biogeography of mosasauroids and
plesiosaurs is beyond the scope of our study; however, for the sake of
discussion related to possible competitive interactions of mosasauroids
and plesiosaurs, we provide a brief insight into possible geographic
origins for mosasauroid and plesiosaur clades. We took the MCCTs
resulting from the Bayesian analyses of both datasets, loaded them to
Mesquite 3.61 (Maddison & Maddison, 2019)
and created a new character matrix with a single new character scored
for each OTU—the continent of discovery. Such demarcation only very
roughly corresponds to the real ancient biogeographic settings (such as
Cretaceous epicontinental seas); however, for the purposes of our study
such highly simplified approach seems to be sufficient as the ancestral
areas in the time interval that is of special interest for us (e.g., the
Western Interior Seaway [covered under ‘North America’] in the
Turonian; see Figs. 1 and 2) do not seem to comprise water bodies with geographically isolated populations.
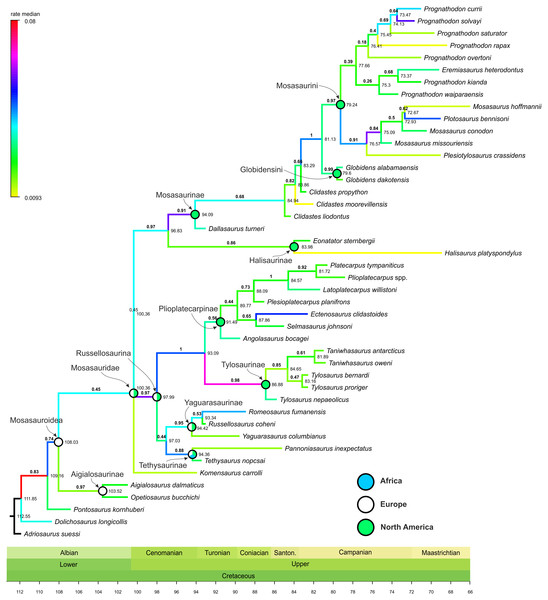
Figure 1: Maximum Clade Credibility Tree (MCCT) of Mosasauroidea.
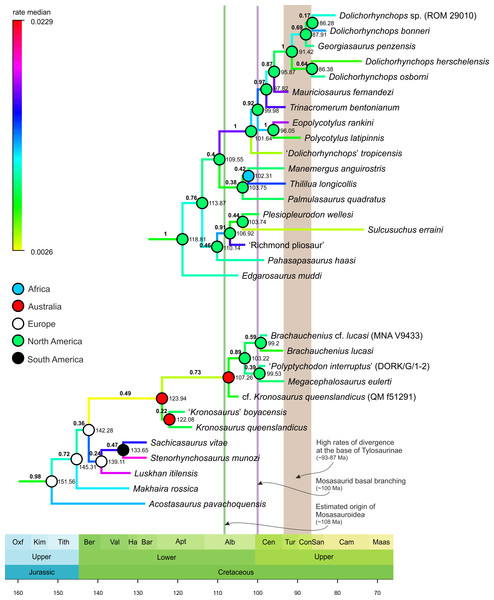
MCCT of Mosasauroidea, rooted on Adriosaurus. Branches colored according to median rate of divergence. Time scale in My. Values above branches (bold) indicate the posterior probability of the clade; values at nodes indicate median age of divergence of node (Ma). Circles on nodes indicate areas of origin reconstructed for major mosasauroid clades.Figure 2: Selected segments of the Maximum Clade Credibility Tree (MCCT) of Plesiosauria.
The topologies and rates of divergence for brachauchenine pliosaurids and polycotylid leptocleidians, with some major events in the evolutionary history of Mosasauroidea. Branches colored according to median rate of divergence. Time scale in My. Values above branches (bold) indicate the posterior probability of the clade; values at nodes indicate median age of divergence of node (Ma). Circles on nodes indicate reconstructed areas of origin.Results
Rates of morphological evolution
The rate of evolutionary divergence inferred among the branches of the mosasauroid MCCT (both internodes and terminal leaves; Fig. 1)
ranges between 0.0093 and 0.0800 changes per branch (median value =
0.0289, 25–75 percentile range: 0.0195–0.0367). During their
evolutionary history mosasauroids experienced intervals of elevated
rates of divergence. High rates are especially apparent at the origin of
Russellosaurina (∼100–98 Mya; rate = 0.0588), the clade consisting of
plioplatecarpines and tylosaurines (∼98–93 Mya; rate = 0.0472), the base
of Tylosaurinae (∼93–87 Mya, rate = 0.0674), and near the base of
Mosasaurinae (∼97–94 Mya; rate = 0.0577). An increase in rates of
morphological evolution is also apparent at the base of the node
comprising Mosasaurus and Plotosaurus (∼77–75 Mya; rate = 0.0588).
Only
three plesiosaur lineages might have affected mosasauroid evolution or
been affected by interactions with them. These include the polycotylid
leptocleidians, elasmosaurids, and brachauchenine pliosaurids. However,
with their elongated necks and long and pointed teeth, elasmosaurids
were substantially distinct from all known members of Mosasauroidea,
thus occupying dissimilar niches (e.g., Massare, 1987). As such, additional comparisons will be limited only to the brachauchenine and polycotylid plesiosaurs (see Fig. 2; due to the large size of the MCCT of Plesiosauria, the full tree is provided as the Supplemental Information VIII).
The
origin of the least inclusive clade formed by Russellosaurina and
Mosasaurinae (+ Halisaurinae) occurred ∼100 Mya, and the splitting of
the clade uniting plioplatecarpines and tylosaurines ∼93 Mya; thus
covering the last few millions of years of the brachauchenine evolution.
In turn, during the splitting of the smallest clade comprising
globidensins and mosasaurins (∼81 Mya) and the origin of the Mosasaurus node (∼75 Mya), pliosaurids were probably already extinct and polycotylids rare (e.g., Sato, 2005; Salgado, Parras & Gasparini, 2007; O’Gorman & Gasparini, 2013; Novas et al., 2015; Fischer et al., 2018; Morgan & O’Keefe, 2019).
The last increase in the rates of morphological evolution within Pliosauridae occurred at the base of the clade formed by Luskhan itilensis, Stenorhynchosaurus munozi, and Sachicasaurus vitae
(∼142–139 Mya) and within that clade. Last brachauchenines of the early
Late Cretaceous (Cenomanian-Turonian) have not experienced significant
oscillations in their evolutionary rates, indicating potential
evolutionary conservatism among the last pliosaurids.
In contrast, polycotylids experienced elevated rates in the ‘middle’ Cretaceous members of the clade.
(See Supplemental Information IX for resulting ‘log’ files from the analyses of both datasets).
Bayesian analysis of plesiosaur phylogenetic relationships
The
phylogenetic relationships of Mosasauroidea inferred by means of
Bayesian analysis of the dataset presented herein have already been
thoroughly discussed in Simões et al. (2017) and Madzia & Cau (2017). Owing to the fact that the overall tree topology of the mosasauroid MCCT is the same as in Madzia & Cau (2017), with minor differences in the mosasauroid outgroup and in the ‘Prognathodon’ and ‘Mosasaurus’ grouping, detailed discussion of the results is provided only for the plesiosaur tree (see Supplemental Information VIII for the full tree).
Inference
of plesiosaur interrelationships through Bayesian analysis, including
estimates of rates of divergence for some clades, has previously been
published as well (Cau & Fanti, 2016); however, the matrix in that study was significantly reduced to include only 39 OTUs. Pliosaurids have been represented as in Benson et al. (2013), the study that Cau & Fanti (2016) took the dataset from, with the only addition being their newly-established pliosaurid taxon Anguanax zignoi.
The representatives of other major clades (rhomaleosaurids and
plesiosauroids) were mostly excluded. Anyway, the original dataset has
been substantially expanded and modified since 2016 (see some of the
recent additions and modifications in ‘Methods’). Furthermore, there are
two substantial methodological differences between the Bayesian
inference of Cau & Fanti (2016) and the one performed in the present study:
-
In the present study, the age uncertainty of each taxon is incorporated in the analysis: age prior of all fossil taxa is defined as a uniform range including the absolute age limits of the shortest stratigraphic range unambiguously including any taxon. This approach differs from that followed in Cau & Fanti (2016), who used for each tip a fixed age prior defined arbitrarily by the mean value of the stratigraphic uncertainty of each taxon.
-
The tree model used here discriminates between anagenetic and cladogenetic patterns of evolution; therefore, it may test whether some of the taxonomic units that are included actually form anagenetic sequences. The analysis in Cau & Fanti (2016) was run on a previous version of BEAST which did not implement the Sampled Ancestor Fossilized Birth Death Skyline Model (Gavryushkina et al., 2014), and thus was a priori constrained to reconstruct exclusively cladogenetic frameworks.
As discussed by Cau (2019), the results of the analysis of Cau & Fanti (2016)
may thus be methodologically biased in potentially inflating the extent
of the inferred ghost lineages and also in pre-dating the ages of the
divergence events.
The
present study includes the first Bayesian analysis of the full dataset,
with most representatives of all major plesiosaur clades, and including
the aforementioned modifications.
The overall tree topology broadly agrees with those reconstructed through more recent parsimony analyses (see, e.g., Ketchum & Benson, 2010; Benson et al., 2013; Benson & Druckenmiller, 2014; Fischer et al., 2015; Cau & Fanti, 2016; Otero, 2016; Sachs, Hornung & Kear, 2016; Fischer et al., 2017; O’Gorman et al., 2017; Sachs, Hornung & Kear, 2017; Serratos, Druckenmiller & Benson, 2017; Fischer et al., 2018; O’Gorman, Gasparini & Spalletti, 2018; Páramo-Fonseca, Benavides-Cabra & Gutiérrez, 2018; Sachs, Lindgren & Kear, 2018; Madzia, Sachs & Lindgren, 2019; Morgan & O’Keefe, 2019). Plesiosauria (posterior probability [pp ] = 1; node origin estimated at ∼241 Mya) basally branches into Rhomaleosauridae (pp = 0.96; ∼215 Mya) and Neoplesiosauria (pp = 0.88; ∼215 Mya), consisting of Pliosauridae (pp = 1; ∼206 Mya) and Plesiosauroidea (pp = 0.89; ∼210 Mya). Within the pliosaurid branch, two nodes have been named—Thalassophonea (pp = 0.59; ∼174 Mya) and Brachaucheninae (pp
= 0.98; ∼152 Mya). Interestingly, contrary to recent studies assessing
the phylogenetic relationships of pliosaurid plesiosaurs by means of
parsimony analyses (e.g., Fischer et al., 2015; Fischer et al., 2017; O’Gorman, Gasparini & Spalletti, 2018; Páramo-Fonseca, Benavides-Cabra & Gutiérrez, 2018; Madzia, Sachs & Lindgren, 2019), the monophyly of Pliosaurus may be considered supported (pp
= 0.83). All ‘major’ plesiosauroid sub-clades appear to be very well
supported by our Bayesian analyses (with possible exception to the basal
branching of Elasmosauridae; see below). The clade Cryptoclidia (pp = 1; ∼180 Mya) consists of Cryptoclididae (pp = 1; ∼176 Mya) and Xenopsaria (pp = 1; ∼158 Mya), which, then, comprises the clade Leptocleidia (pp = 1; ∼145 Mya)—including Leptocleididae (pp = 0.9; ∼140 Mya) and Polycotylidae (pp = 1; ∼119 Mya)—and its closest relatives (Brancasaurus and ‘Gronausaurus’), and the clade Elasmosauridae (pp = 0.44; ∼144 Mya). However, the low pp value for the base of Elasmosauridae might be due to the problematic placement of Lagenanectes richterae which might be a non-elasmosaurid xenopsarian (D. Madzia, unpublished results). The pp value of the more ‘traditional’ elasmosaurid grouping (that is, exclusive of L. richterae) is very high (pp = 0.99).
Discussion
Estimates of evolutionary rates and potential biases
The impact of phylogenetic uncertainties on inferences of evolutionary rates
Both
mosasauroid and plesiosaur datasets include shortcomings that might
have had an impact on the inferences of the rates of morphological
evolution within these groups. The dataset used for the assessment of
the phylogenetic relationships within mosasauroids has been reviewed and
discussed by Madzia & Cau (2017)
who recommended that the currently inferred topologies should be seen
as tentative pending extensive modifications to the data sampling.
Nevertheless, Madzia & Cau (2017)
also noted that analyses using multiple phylogenetic methods revealed
general congruencies regarding monophyly of major mosasauroid groups
(mosasaurines, tylosaurines, plioplatecarpines, halisaurines,
tethysaurines, and possibly also yaguarasaurines). Differences involved
their mutual relationships which remain largely unsettled. At the same
time, elevated rates of morphological evolution have been inferred for
well-supported clades with posterior probability (pp) values around 0.9 and often higher (Plioplatecarpinae + Tylosaurinae: pp = 1; Tylosaurinae: pp = 0.98; Mosasaurinae: pp = 0.91; Globidensini + Mosasaurini: pp = 1; Mosasaurus: pp = 0.84; see Fig. 1).
Given such results, it seems probable that the rates inferred for major
or well-supported mosasauroid nodes would stay similar even after the
changes to the dataset suggested by Madzia & Cau (2017).
Similarly,
there do not seem to be any doubts regarding the composition of
advanced brachauchenines and polycotylids, the two groups that are
compared here with mosasauroids.
Sampling bias
Any
inferences of the rates of morphological evolution by means of Bayesian
phylogenetics can or should be based only on reasonably complete
material. That means, for example, that isolated fragments often have to
be omitted from the datasets as the methods cannot handle them or may
produce dramatically labile relationships. Still, they might provide
important information on the ‘persistence’, diversity, and geographic
distribution of particular lineages. For instance, fragmentary material
suggests that the brachauchenine lineage might have reached the latest
middle Santonian (∼84 Ma), as might be evidenced by an isolated tooth
crown originating from the Micraster coranguinum Zone (the
uppermost lower Coniacian to the uppermost middle Santonian) of the
Seaford Chalk Formation, Gravesend, Kent, England (Madzia, 2016).
Nevertheless, the material is too limited to serve as an indicator of
the lineage ‘vitality’ and competitiveness. Instead, if really belonging
to a brachauchenine (and not a robust-toothed polycotylid), it seems to
merely represent a relict of the once widespread pliosaurids. In turn,
other isolated elements belonging to pliosaurids and polycotylids, such
as those studied by Kear et al. (2014), Madzia & Machalski (2017), Sachs et al. (2017), Sachs et al. (2018), Sachs et al. (2020), and Zverkov & Pervushov (2020)
might suggest a higher taxic diversity and a wider geographic
distribution of the clades during the Albian–Turonian (‘middle’
Cretaceous).
Similarly, the large-scale assessment of pliosaurid teeth by Zverkov et al. (2018),
that was based on material representing the vast majority of pliosaurid
taxa, including assemblages of isolated teeth, suggested that
pliosaurids (1) could have been represented by two lineages in the
Cretaceous, instead of one, and that (2) they experienced the highest
dental disparity around the Jurassic/Cretaceous boundary interval. Such
results were surprising because they were and still are in striking
contrast with all studies assessing the phylogenetic relationships of
Pliosauridae, that cannot consider numerous latest Jurassic/Early
Cretaceous specimens due to their highly fragmentary nature, and that
suggest the presence of a single clade of pliosaurids crossing the
Jurassic/Cretaceous boundary. Newer studies, such as that of Lukeneder & Zverkov (2020) provide further support for the findings of Zverkov et al. (2018).
Comparing mosasauroid and plesiosaur traits
Tooth crown morphologies and trophic guilds
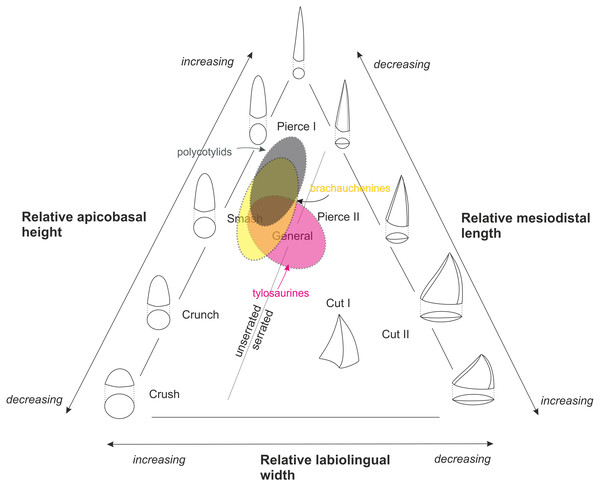
Driven by “a lack of precision in correlating tooth type and preferred prey” in Mesozoic marine amniotes, Massare (1987)
designed seven trophic guilds based on tooth crown morphologies: (I)
crush, (II) crunch, (III) smash, (IV) pierce I, (V) pierce II, (VI)
general, and (VII) cut (Massare, 1987: 130–131), and provided characteristics of particular crown morphologies used in determining assignment to guilds (Massare, 1987: 132, Table 3). Her classification has been further modified by Hornung & Reich (2015)
who divided the ‘cut’ guild into two categories, ‘cut I’ and ‘cut II’,
to distinguish between taxa with labiolingually expanded crown with
cutting edges (‘cut I’) from those with strongly compressed and
blade-shaped teeth (‘cut II’) (see Fig. 3). Even though the guild classification proposed by Massare (1987) has not been accepted universally (Buchy, 2010), recent quantitative studies using teeth of marine amniotes to evaluate feeding ecologies validated such system (e.g., Foffa et al., 2018).
Figure 3: Massare’s (1987) ternary graph, as modified by Hornung & Reich (2015), showing the association of tooth crown morphologies and function.
An approximate partial overlap of the tylosaurine dental morphospace with that of brachauchenine pliosaurids and polycotylid leptocleidians.
The
Late Cretaceous brachauchenines and polycotylids can be categorized
relatively easily within that system. With their conical and slightly
curved teeth, both these clades belong to the non-carinate/unserrated
‘general’/‘smash’/‘pierce I’ guilds, though polycotylids usually possess
less robust teeth leaning further towards the ‘pierce I’ part of the
system (Fig. 3).
The guild assignment of the mosasauroids is problematic due to the
apparent and widespread pseudoheterodonty in some clades. Specifically,
the shape of the mosasauroid teeth often differs depending on the
positions of the teeth in jaws (see, e.g., LeBlanc, Caldwell & Bardet, 2012; Otero et al., 2016b; Madzia, 2019). For example, the derived mosasaurine mosasaurid Mosasaurus lemonnieri,
which is known from a number of well-preserved specimens with
reasonably complete jaws, shows anterior-to-middle tooth crowns with
subcircular/ovoid cross-sections and carinae variably developed either
on a short apical segment of the tooth crowns (in IRSNB R 366, 368, 369)
or along their entire apicobasal length (serrated only in the somewhat
problematic specimen IRSNB R 377). Its posteriorly positioned teeth, in
turn, tend to be increasingly labiolingually compressed (Madzia, 2019).
Further differences are appearing through the ontogeny as larger
(presumably older) individuals appear to show more robust teeth. Such
morphological diversity of its tooth crowns makes the taxon occupying a
wide field of the ternary graph (from ‘pierce I’/‘pierce II’ through the
‘cut I’/‘cut II’ guilds). In tylosaurines, in turn, the teeth occupy
the ‘smash’ to ‘general’/‘pierce II’ field, and mosasaurins show dental
morphologies indicative of the ‘crunch’/‘general’ to ‘cut I’/‘cut II’
guilds (Schulp et al., 2006; Ross, 2009; Hornung & Reich, 2015).
Therefore, many mosasauroids could likely occupy the same trophic
levels of generalists and represent the same trophic guilds as
robust-toothed short-necked plesiosaurs.
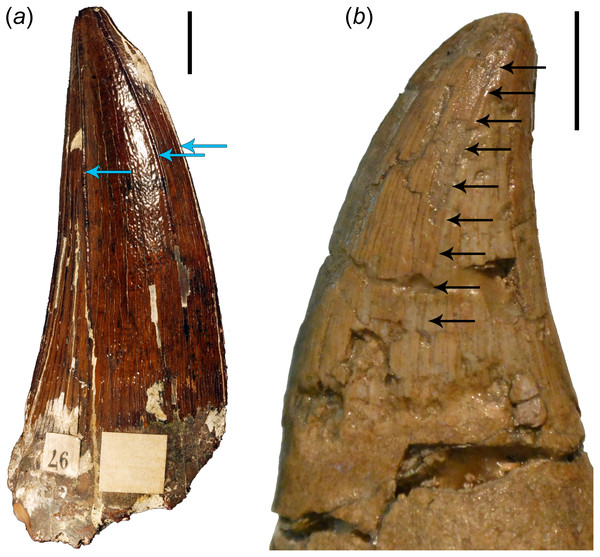
It
is also essential to note here that even though most mosasauroids
possess carinate teeth (which distinguishes them from the teeth of the
last brachauchenines as well as polycotylids), their distal carinae are
often displaced labially (see Fig. 4),
especially in the teeth from the anterior to middle section of the
jaws. In such cases, the distal carinae resemble (and are often less
pronounced than) the apicobasal ridges observable in plesiosaur teeth
(see Fig. 4 and the distribution of apicobasal ridges in brachauchenine teeth in Madzia (2016), discussion of outer enamel structural elements in pliosaurid teeth by Zverkov et al. (2018), and assessment of ridge evolution and function in marine amniotes by McCurry et al. (2019)) and certainly does not play the role of a cutting edge (as in labiolingually strongly compressed tooth crowns).
Figure 4: Comparisons of brachauchenine and tylosaurine tooth crowns.
(a) Isolated tooth crown (CAMSM B 57378) attributed to the dubious brachauchenine taxon ‘Polyptychodon interruptus’ (described and pictured by Madzia, 2016), from the Cambridge Greensand Member of the West Melbury Marly Chalk Formation in labial view; showing the distribution and development of the apicobasal ridges (indicated by blue arrows); and (b) left dentary tooth crown (ld04) of IRSNB R 23 (type of Tylosaurus bernardi) in labial view. Arrows on (b) show a labial displacement of the distal carina; resembling the apicobasal ridges in plesiosaurs. Scale bars = 1 cm. Photograph credits: Daniel Madzia.Body size evolution
The earliest mosasauroids, such as Aigialosaurus, Carsosaurus, Haasiasaurus, Komensaurus, and Opetiosaurus,
had slim and elongated bodies, with a total length of about 1 to 2 m,
which strongly contrasts with the bulky, multitone contemporary
plesiosaurs. However, larger-sized mosasauroids appeared relative early
in the evolutionary history of the clade, as is documented, for example,
by the specimen TMM 43345-1, which represents a large tylosaurine (Bell, Barnes & Polcyn, 2013).
The specimen originates from the upper middle Turonian of the Ernst
Member (Boquillas Formation) of Texas; thus, tylosaurines evolved
larger-sized forms around the time or shortly after brachauchenines
became extinct. Nevertheless, members of other mosasauroid clades
(plioplatecarpines and mosasaurines) evolved large sizes (>5 m in
length) relatively early as well (see, e.g., Polcyn et al., 2014: Appendix A. Supplementary data).
Evolution of swimming abilities
Swimming
modes and abilities of Mesozoic aquatic vertebrates are tightly
connected with their physiology, behavior, and other aspects of their
biology and, thus, constitute an important research area (e.g., Massare, 1988; Massare, 1994; Motani, 2002).
A number of studies have thoroughly assessed the swimming abilities in
plesiosaurs and discussed the differences in the two main plesiosaur
‘body plans’ –the ‘long-’ and ‘short-necked’ forms (e.g., Frey & Riess, 1982; Tarsitano & Riess, 1982; Godfrey, 1984; Halstead, 1989; Nicholls & Russell, 1991; Lingham-Soliar, 2000; O’Keefe, 2001; Carpenter et al., 2010; Liu et al., 2015; Muscutt et al., 2017; Noè, Taylor & Gómez-Pérez, 2017; Troelsen et al., 2019).
The same applies to mosasauroids whose swimming abilities and
especially their origin have been assessed through detailed studies of
various aspects of their anatomy (see, e.g., Lindgren, Jagt & Caldwell, 2007; Lindgren et al., 2010; Lindgren, Polcyn & Young, 2011; Konishi et al., 2012; LeBlanc, Caldwell & Lindgren, 2013; Lindgren, Kaddumi & Polcyn, 2013; Houssaye & Bardet, 2013; Cuthbertson et al., 2015; D’Emic, Smith & Ansley, 2015).
The mode of swimming in the two clades is known to have differed
greatly. Plesiosaurs have exhibited a limb-based propulsion while
mosasauroids employed lateral undulatory locomotion. These differences
also apparently reflect the modes of predation in these groups. Owing to
their anatomical similarities—large heads, relatively short necks,
bulky bodies, and rather short tails—brachauchenines and polycotylids
were specialized for maneuverability and pursuit (e.g., O’Keefe, 2001).
Mosasauroids, in turn, had long been characterized as being
slower-swimming predators adapted for brief ambush pursuits (e.g., Massare, 1988; Massare, 1994; Motani, 2002).
Over the last few years, however, the knowledge of the mosasauroid body
plan evolution has improved considerably (see, e.g., discussions in Lindgren, Polcyn & Young (2011) and Lindgren, Kaddumi & Polcyn (2013), suggesting that the swimming performance of derived mosasauroids was similar to that of pelagic sharks.
In general, mosasauroids comprised a wide array of taxa; from semi-aquatic forms (e.g., Bell & Polcyn, 2005; Polcyn & Bell, 2005; Dutchak & Caldwell, 2006; Dutchak & Caldwell, 2009; Caldwell & Palci, 2007; Mekarski et al., 2019) to fully aquatic swimmers (see, e.g., Lindgren, Jagt & Caldwell, 2007).
Nevertheless, the course and timing of their transition from semi- to
fully aquatic morphologies (that is, from ‘plesiopedal-plesiopelvic’ to
‘hydropedal-hydropelvic’ conditions; sensu Bell & Polcyn (2005) and Caldwell & Palci (2007),
a key aspect when considering potential competitive interactions
between mosasauroids and plesiosaurs, is somewhat hindered by
conflicting hypotheses of the early evolution of the group. Current
phylogenetic assessments of the mosasauroid basal branching are highly
dependable on the tree-search strategies used (Simões et al., 2017; Madzia & Cau, 2017). For instance, out of several phylogenetic methods applied, only the parsimony analysis with implied weighting performed by Simões et al. (2017), with the default setting of the concavity parameter (K = 3),
inferred a single origin of the fully aquatic lifestyle in mosasauroids
(with a reversal to the ‘plesiopelvic’ condition in tethysaurines). Madzia & Cau (2017) questioned these findings on the ground of the ongoing debate regarding the meaning of the K parameter (O’Reilly et al., 2016; Congreve & Lamsdell, 2016; Goloboff, Torres & Arias, 2018) and also the lack of multiple approaches to the phylogenetic assessment using the implied weighting function (see Goloboff, 1993; Goloboff, 1995; Goloboff et al., 2008; Goloboff, Torres & Arias, 2018). It is also essential to note that the use of the K-value that is set as default in TNT (that is, K = 3), appears to be too strong and leading to unnatural grouping of OTUs, especially for larger datasets (Goloboff, Torres & Arias, 2018). Thus, higher (though not too high) values should be preferred (see also discussion in Herne et al., 2019: Supplemental text S1: 9–12]).
Considering the results stemming from the most recent parsimony and Bayesian analyses (Simões et al., 2017; Madzia & Cau, 2017;
this study), mosasauroids might have evolved the fully aquatic
lifestyle more than once. Still, the course of the transition remains a
subject for detailed multidisciplinary assessments. For example, despite
that the study of Houssaye et al. (2013)
was focused on mosasaurine mosasauroids, the authors analyzed the
limb-bone osteohistology in a wide variety of taxa, including the basal
mosasaurine Dallasaurus, a taxon with a
‘plesiopedal’/‘hydropelvic’ morphology that is ‘transitional’ between
semi- and fully-aquatic forms, as well as specimens assigned to the
fully aquatic (‘hydropedal’/‘hydropelvic’) taxa Clidastes, Globidens, Mosasaurus, Plotosaurus, and ‘Prognathodon’. The results, when further compared with previous osteohistological studies (e.g., Houssaye & Bardet, 2013),
revealed that ‘transitional’ mosasauroids, or at least those
representing forms intermediate between basal semi-aquatic mosasauroids
and advanced fully-aquatic mosasaurines, exhibited a peculiar inner bone
organization characterized by the combination of terrestrial-like and
aquatic features that suggested a more gradual adaptation to open marine
environments than previously thought. Interestingly, the acquisition of
the ‘hydropedal’ and ‘hydropelvic’ conditions, as inferred through our
Bayesian analysis, occurred approximately at the time and within the
lineages with the highest rates of evolution, resulting in the
appearance of good swimmers around the time the brachauchenines
experienced low rates of morphological evolution and died out.
Thermoregulation and metabolic rates
Oxygen isotope compositions (δ18O)
data obtained from the tooth phosphate of plesiosaurs suggest that they
were able to regulate their body temperature independently of the
surrounding waters and had high metabolic rates that are required for
fast swimming over large distances and predation (Bernard et al., 2010). Plesiosaur metabolic rates have been later independently assessed through the study of their osteohistology (Fleischle, Wintrich & Sander, 2018),
which supported the inference of high rates in the clade. In
mosasauroids, the available evidence offers slightly ambiguous results. Bernard et al. (2010)
proposed that the body temperature of mosasauroids could have been at
least partly influenced by ambient conditions; still, they found support
for high metabolic rates in mosasauroids. While reassessing the results
of Bernard et al. (2010) and Motani (2010) noted that the temperatures provided in Bernard et al. (2010) might be artifacts arising from time-dependent depletion of δ18O (see also Veizer, Godderis & François, 2000),
and argued that mosasauroids might have had a tendency to overheat,
proposing that they may have been gigantothermic. Nevertheless, such
conclusions appear to be in disagreement with a further stable oxygen
isotope study (Harrell, Pérez-Huerta & Suarez, 2016)
that characterized mosasauroids as being endotherms rather than
gigantotherms. With respect to the mosasauroid metabolic rates, Houssaye et al.’s (2013)
osteohistological study showed that their basal metabolic rates were
intermediate between those of the extant leatherback turtles (that are
homeothermic but not endothermic; e.g., Motani (2010) and Houssaye (2013) and those inferred for plesiosaurs (that are endothermic e.g., Bernard et al., 2010; Motani, 2010; Houssaye, 2013; Fleischle, Wintrich & Sander, 2018; Fleischle et al., 2019).
Reproduction and early life history
Available
evidence related to the reproductive strategies and early life history
in mosasauroids and plesiosaurs is currently limited to a few studies
(e.g., Caldwell & Lee, 2001; Kear, 2007; O’Keefe & Chiappe, 2011; Houssaye & Tafforeau, 2012; Houssaye & Bardet, 2013; Field et al., 2015) and reports that have not been published beyond conference abstracts (e.g., Bell et al., 1996; Everhart, 2002; Bell & Sheldon, 2004). A study describing the first gravid plesiosaur, a polycotylid specimen referred to Polycotylus latipinnis (O’Keefe & Chiappe, 2011),
has initiated comparisons between reproductive strategies of
plesiosaurs and other marine amniotes, including mosasauroids. Both
clades, mosasauroids and plesiosaurs, have been viviparous though their
reproductive strategies differed. In the early (semi-aquatic)
mosasauroid Carsosaurus, females have been apparently giving birth to at least four progenies (Caldwell & Lee, 2001).
Published record does not provide definitive answer regarding the
number of embryos in more advanced (larger and fully aquatic) members of
Mosasauroidea though preliminary reports suggested that
plioplatecarpines were giving birth to multiple progenies as well (Bell & Sheldon, 2004). The gravid plesiosaur specimen, in turn, shows only a single fetus (O’Keefe & Chiappe, 2011).
Following comparisons of the traits observed in that specimen to those
in the closest extant ecological analogs (odontocete cetaceans) and taxa
with some plesiosaur-like reproductive traits (Egernia spp.), O’Keefe & Chiappe (2011) suggested that plesiosaurs were K-selected
and hypothesized that they were social and may have been engaged in
parental care. It could be speculated that multiple progenies in
mosasauroids, if also present in large-sized forms, might have given
these squamates some advantages over plesiosaurs, especially if they
were born in open pelagic setting and immediately occupied it (e.g., Houssaye & Tafforeau, 2012; Field et al., 2015). When such things are considered, it is worth noting, however, that the theory of r/K-selection of MacArthur & Wilson (1967), a paradigm popular as a predictive model for life-history evolution in the late 1960s and 1970s (see also Pianka, 1970), has long been challenged (see, e.g., discussion in Reznick, Bryant & Bashey, 2002).
Even if mosasauroids and plesiosaurs differed in both, their
reproductive strategies and early life history, the evolutionary meaning
of these differences and their impact on the life history of
mosasauroids and plesiosaurs, when assessed from the perspective of
their niche occupation, remains unknown.
The record of interactions between mosasauroids and plesiosaurs
It
is beyond doubt that sympatric mosasauroids and plesiosaurs interacted
at the individual scale. Direct evidence, however, is scarce. Everhart (2004) published on a partial plesiosaur specimen preserved as the stomach contents of an 8.8-meter-long adult of Tylosaurus proriger.
It was discovered in 1918 in the lower Campanian strata of the Niobrara
Formation, near Twin Butte Creek, Logan County, Kansas, and first
mentioned by Sternberg (1922) but it has not been properly described until 2004. Everhart (2004) admitted a poor state of preservation of the plesiosaur remains but suggested that they most likely belong to the polycotylid Dolichorhynchops osborni. Einarsson et al. (2010),
in turn, described a plesiosaur propodial of latest early Campanian
age, discovered at the Åsen locality, Kristianstad Basin, Sweden. Though
incomplete, the specimen was identified as an indeterminate
polycotylid. It possesses a distinctive bite mark that was interpreted
by Einarsson et al. (2010) to be caused by a large mosasaurine comparable to Dollosaurus.
Nevertheless, none of these finds could have been unequivocally
inferred as representing either predatory or scavenging behavior.
Further finds, of interest with respect to early mosasauroid
diversification patterns, include mosasauroid and brachauchenine
specimens that overlap in time and space (see specimens discussed in Martin & Stewart, 1977; Polcyn et al., 2008; Schumacher, 2011; Kear et al., 2014; Everhart, 2016). These discoveries, however, do not show any evidence of direct interactions between the members of the two clades.
Concluding remarks
We
provide the first estimates of the evolutionary rates for mosasauroid
and plesiosaur clades and use the results as a baseline for discussion
and comparisons of traits that might have had some impact on the shape
of the niche structures in Late Cretaceous seas. Owing to the known
stratigraphic distribution of the mosasauroid and plesiosaur lineages,
only three plesiosaur clades might have competed with mosasauroids; the
elasmosaurids, brachauchenine pliosaurids, and polycotylid
leptocleidians. However, considering the overall body plans of the taxa
belonging to these groups, and their tooth crown morphologies, which are
key indicators of dietary niche partitioning (e.g., Massare, 1987; Schulp et al., 2013; Hornung & Reich, 2015; Foffa et al., 2018), we suggest that only brachauchenines and polycotylids might have been possible niche competitors of mosasauroids.
With
respect to the possible competitive interactions between mosasauroids,
brachauchenines, and polycotylids, three hypotheses were considered:
-
First mosasauroids diversified following competition with plesiosaurs.
-
At least some mosasauroids competed with contemporary plesiosaurs or seized the opportunity and occupied their niches when they were in demise or became extinct.
-
The fates of plesiosaurs and mosasauroids were independent of each other (no suggested competitive interactions between mosasauroids and plesiosaurs).
Having
the results of our Bayesian analyses in mind, we have focused on
several traits related to niche occupation. Specifically, we have
compared the body size and swimming abilities of mosasauroids and
plesiosaurs, discussed the thermoregulation and metabolic rates in these
groups, and considered their reproductive strategies and early life
history. Available evidence shows that the earliest mosasauroids
differed from plesiosaurs in all these traits. Earliest Late Cretaceous
(Cenomanian–Turonian) plesiosaurs were large generalists, excellent
swimmers, giving birth to a single large progeny, and with metabolic
rates higher than those of contemporary mosasauroids. In turn, the first
mosasauroids were small, semi-aquatic animals that gave birth to
multiple offspring. These marked differences, however, began to blur in
the Turonian. Around the time the last brachauchenines died out (middle
Turonian; though see Madzia (2016)
for possible younger occurrences) or immediately after the demise of
the clade, mosasauroids experienced high evolutionary rates; they
evolved first larger-sized taxa and apparently also first good swimmers
(e.g., Bell, Barnes & Polcyn, 2013; Polcyn et al., 2014).
No evidence suggests substantial changes in the metabolic rates or
reproductive strategies of larger-sized mosasauroids. In fact,
preliminary reports seem to indicate that even larger mosasauroid taxa
gave birth to multiple progenies (e.g., Bell & Sheldon, 2004).
Still, we refrain from drawing any far-going conclusions based on the
available data as the evolutionary meaning of the discussed differences
is unknown.
Considering
the record of direct mosasauroid-plesiosaur interactions, the fates of
plesiosaurs and mosasauroids were probably not directly independent of
each other. At least two mosasauroid groups containing large taxa, the
tylosaurines and advanced mosasaurines, clearly interacted with
polycotylids (Everhart, 2004; Einarsson et al., 2010).
However, the assertion that the extinction of the last brachauchenines
had been accelerated by the diversification of the tylosaurine
russellosaurinans or that tylosaurines competed for and eventually
‘usurped’ the niche previously occupied by pliosaurid plesiosaurs would
be speculative. The early radiation of polycotylids during the
Early/Late Cretaceous transitional interval apparently produced a burst
of disparity in the clade (Fischer et al., 2018), which could have had an impact on brachauchenines as well. Fischer et al. (2018)
noted that the increased disparity was not an aftermath of the
extinction of ichthyosaurs and pliosaurids, and that the vanishing of
the high disparity in polycotylids during and after the Turonian is
consistent with a model of ‘early experimentation/late constraint’.
Considering the suspiciously thalassophonean-like body plans in the
mid-Cretaceous polycotylid plesiosaurs, such as Edgarosaurus muddi, Mauriciosaurus fernandezi, and Plesiopleurodon wellesi,
that co-occurred with the last pliosaurids (at a time pliosaurids show
low evolutionary rates), it is possible, nevertheless, that competition
with polycotylids could have contributed to the extinction of
Pliosauridae.
The
potential role of mosasauroids in re-shaping the early Late Cretaceous
marine environments is unclear. However, we speculate that the demise of
brachauchenines and decrease in both, phylogenetic diversity and
disparity in polycotylids around the time mosasauroids experienced high
evolutionary rates, might have resulted in that some mosasauroids, such
as tylosaurines, seized the opportunity and inhabited the niche
previously occupied by robust-toothed short-necked plesiosaurs. However,
further clade- and trait-specific studies are necessary in order to
elucidate the patterns of niche occupation in Late Cretaceous seas.
Supplemental Information
Mosasauroid and plesiosaur FADs and LADs and the geographic distribution
Character list for the phylogenetic analyses of Mosasauroidea
Character list for the phylogenetic analyses of Plesiosauria
Maximum Clade Credibility Tree (MCCT) of Plesiosauria
Full phylogenetic tree.
Log files from the Bayesian analyses of Mosasauroidea and Plesiosauria
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Daniel Madzia and Andrea Cau
conceived and designed the experiments, performed the experiments,
analyzed the data, prepared figures and/or tables, authored or reviewed
drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The data matrices used for Bayesian analyses and resulting log files are available in the Supplemental Files.
The data matrices used for Bayesian analyses and resulting log files are available in the Supplemental Files.
Nenhum comentário:
Postar um comentário
Observação: somente um membro deste blog pode postar um comentário.